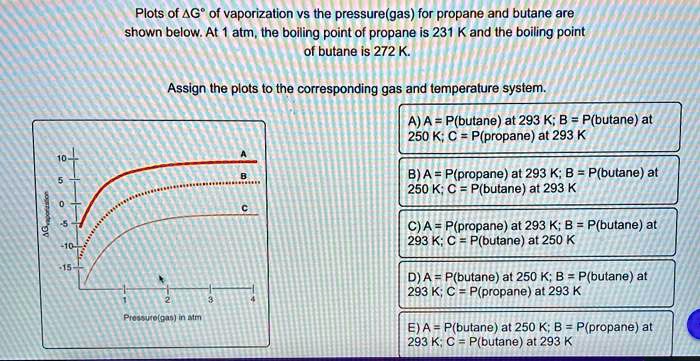
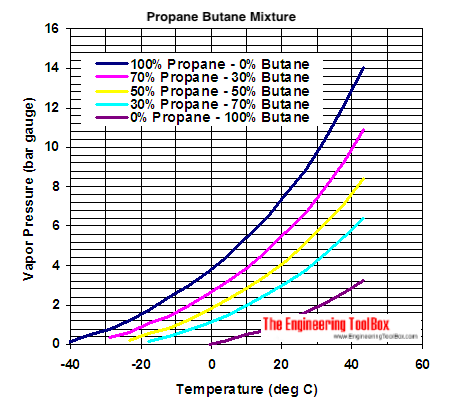
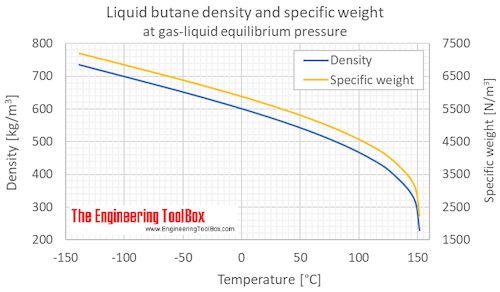
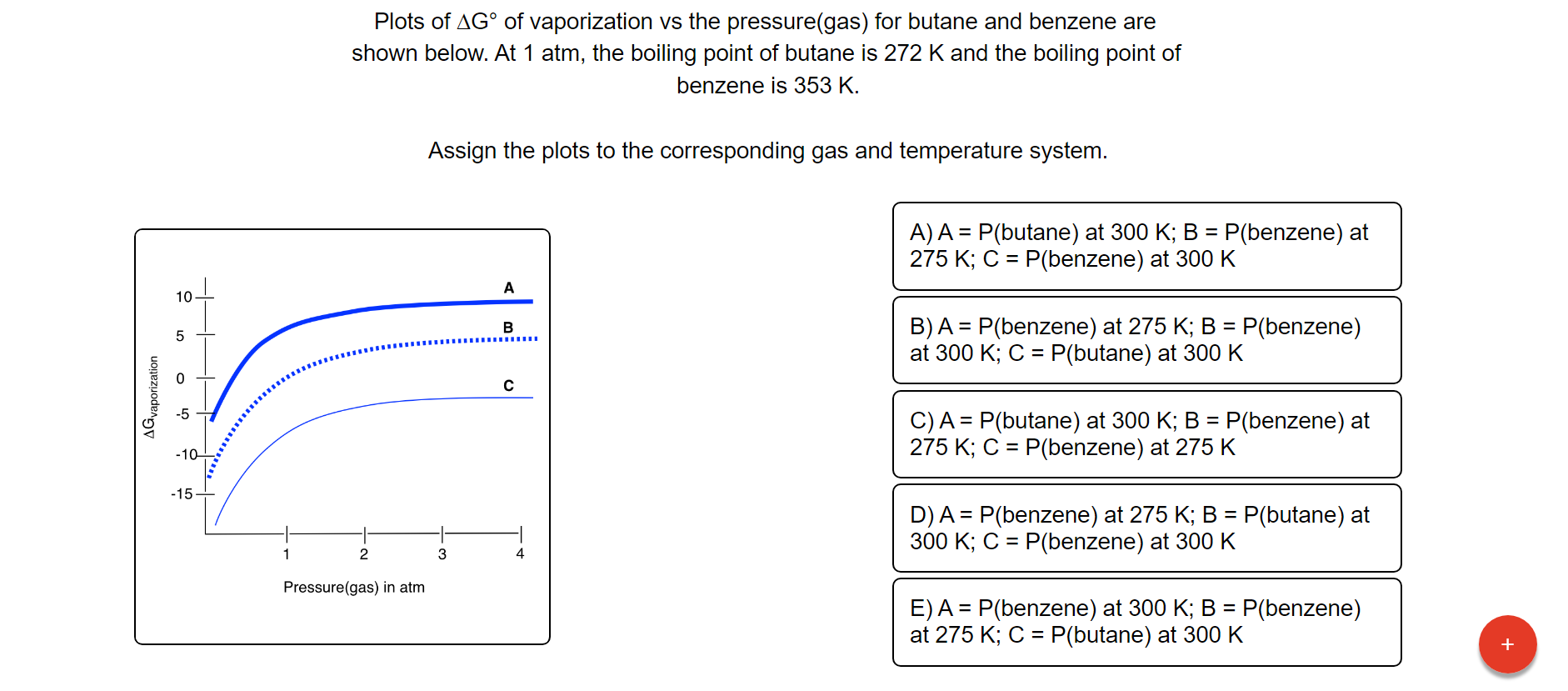
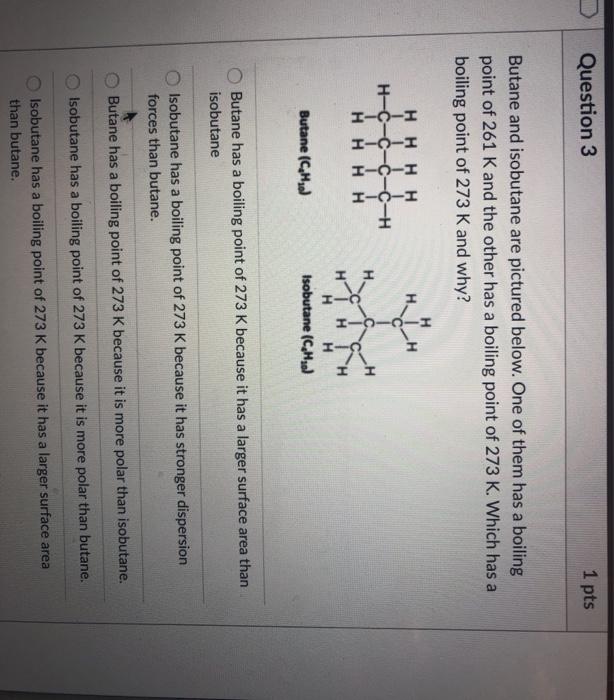
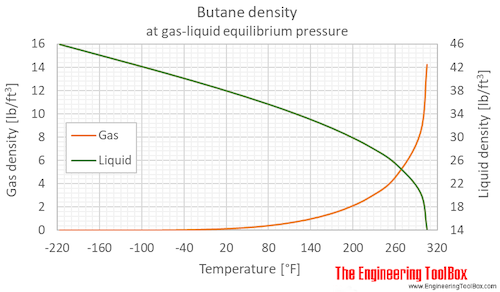
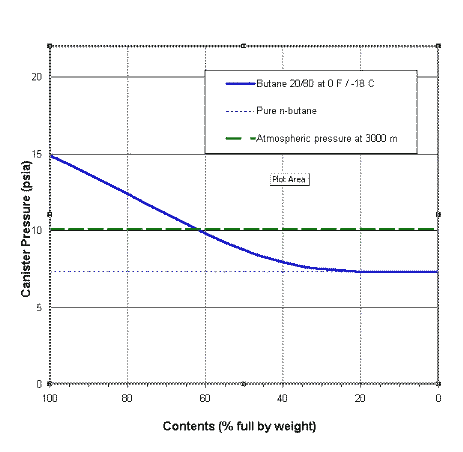
thermodynamics - Are there other properties besides lower boiling point that make isobutane a better refrigerant than butane? - Physics Stack Exchange
Although butane has 4 carbon atoms, its boiling point is lower than compounds that have 3 carbon atoms. Why? - Quora

Consider the following data for butane, C_4H_(10). Normal melting point is -138^(\circ)C. Normal boiling point is 0^(\circ)C. Critical temperature is 152^(\circ)C. Critical pressure is 38 atm. Assume that the triple point is
At 25.0°C, the vapor pressure of butane is 2.30 ATM. What is the pressure in the container at 144.0°C? - Quora

OneClass: 4. n-butane and isobutane are constitutional isomers. What is the boiling point of each com...