Thermo-mechanics aspects of isochoric cryopreservation: A new modeling approach and comparison with experimental data | PLOS ONE
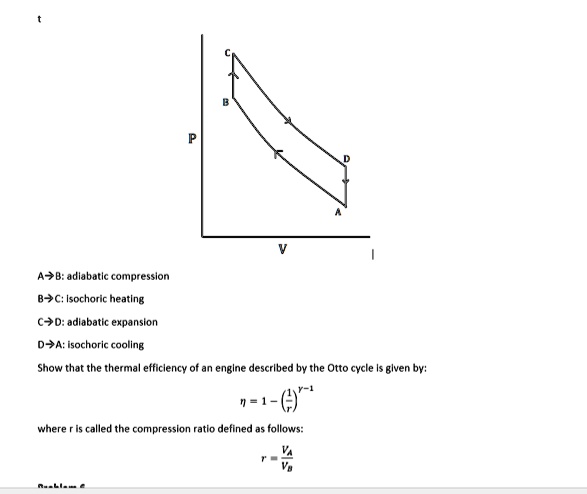
SOLVED: A->B: Adiabatic compression C: Isochoric heating C->D: Adiabatic expansion D->A: Isochoric cooling Show that the thermal efficiency of an engine described by the Otto cycle is given by: η = 1 - (

Stability of Protein Formulations at Subzero Temperatures by Isochoric Cooling - Journal of Pharmaceutical Sciences

File:PV diagram of two paths of isobaric cooling and isochoric heating with same start and end states.svg - Wikimedia Commons

6: Isochoric lines in the pressure-temperature diagram of hydrogen. The... | Download Scientific Diagram
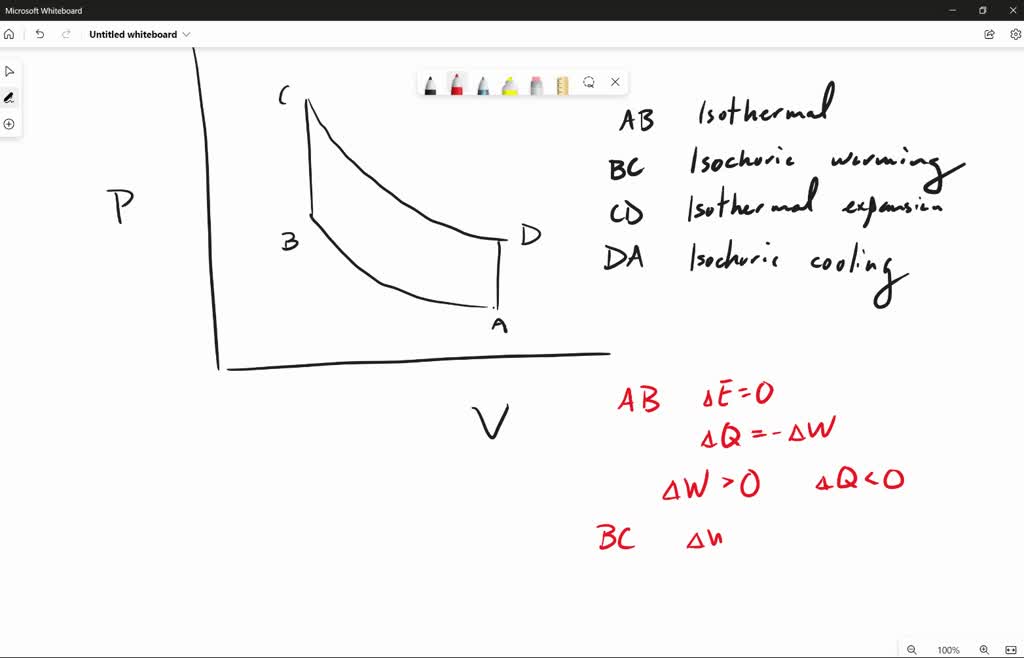
SOLVED: 3. The ideal gas in a closed Stirling process includes four transformations: A - B: Isothermal compressibility B-C: Isochoric warming C-D: Isothermal expansion D -A: Isochoric cooling a) Draw the (V,
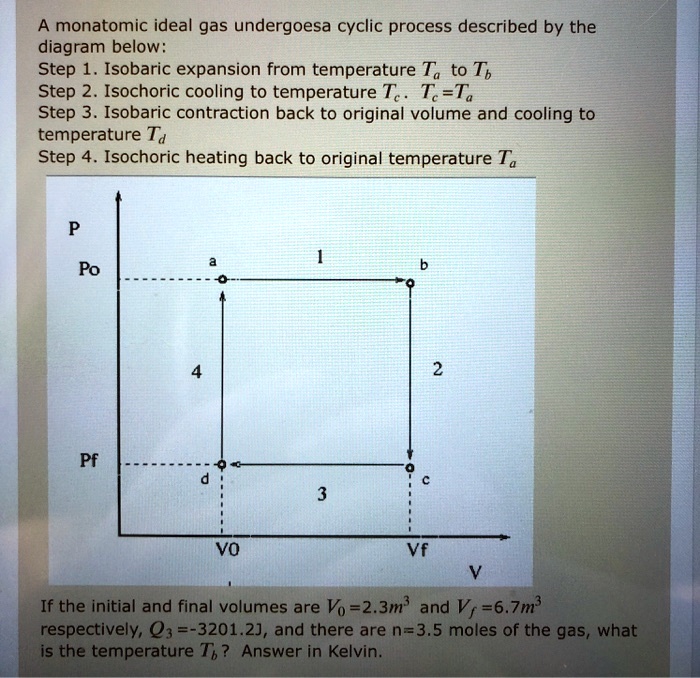
SOLVED: A monatomic ideal gas undergoes a cyclic process described by the diagram below: Step 1: Isobaric expansion from temperature Ta to Tb. Step 2: Isochoric cooling to temperature Tc. Tc =









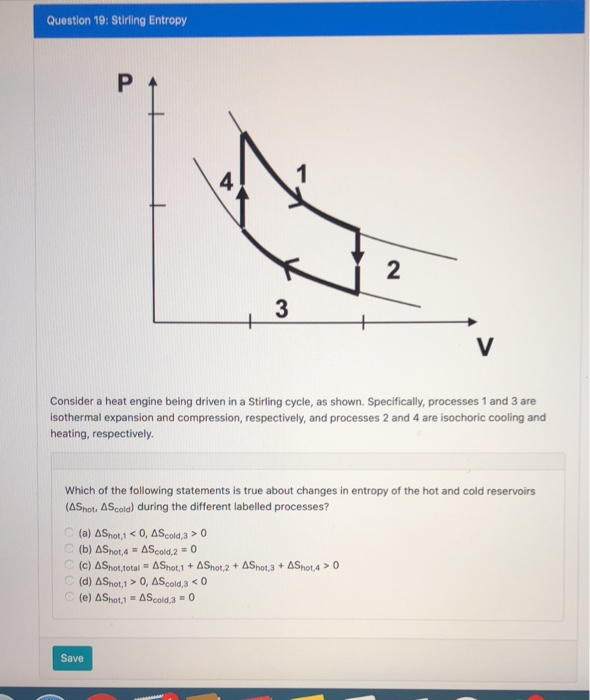
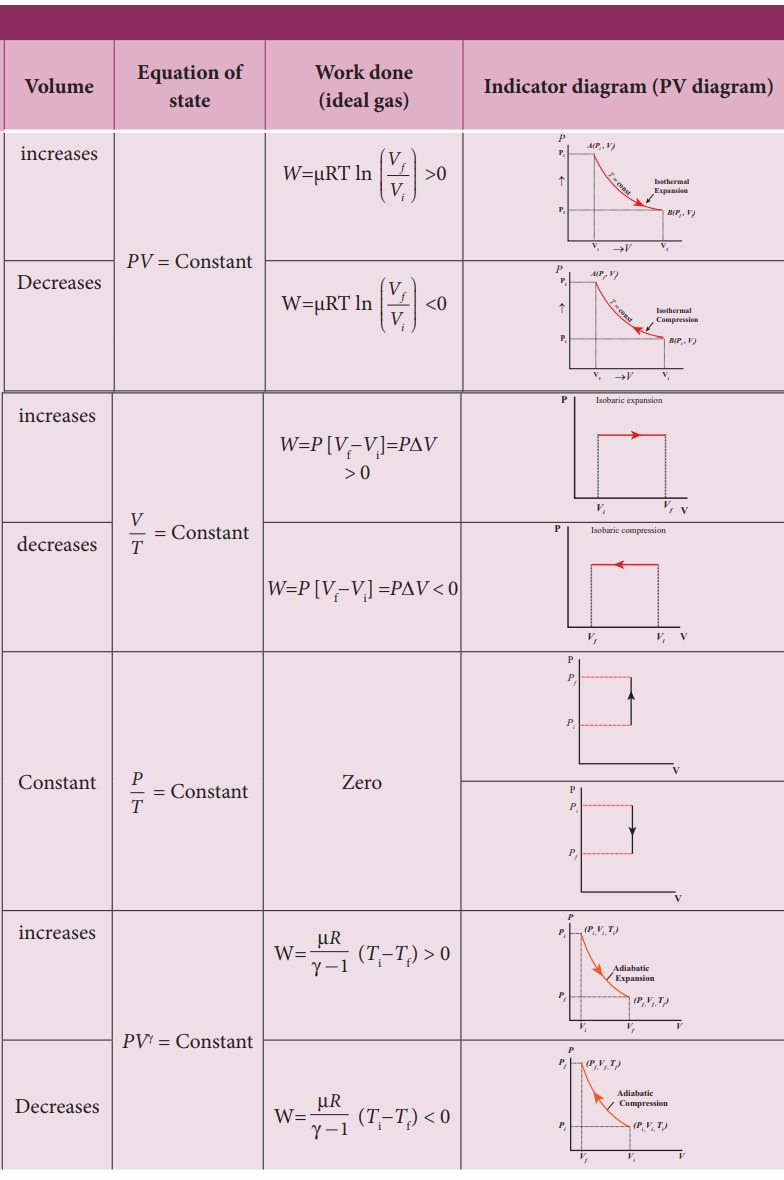
![Isochoric pressure search method [27]. | Download Scientific Diagram Isochoric pressure search method [27]. | Download Scientific Diagram](https://www.researchgate.net/publication/332396195/figure/fig2/AS:747149553827841@1555145743317/Isochoric-pressure-search-method-27.jpg)
